CRO
Managing trials in various regions

Medical Writing
Concept sheets / Protocol Synopses
Study protocols
Investigator Brochures

Pharmacovigilance
Local PV Services
SAE Processing
Scientific Literature Screening
Regulatory Intelligence on PV
Document Preparation & Maintenance
Safety Reporting

Medical Devices
MarPe specializes in medical device and diagnostic clinical development. Our medical, regulatory and operational experts work collaboratively with your team to design and conduct clinical trials in MENA and Africa region
More Details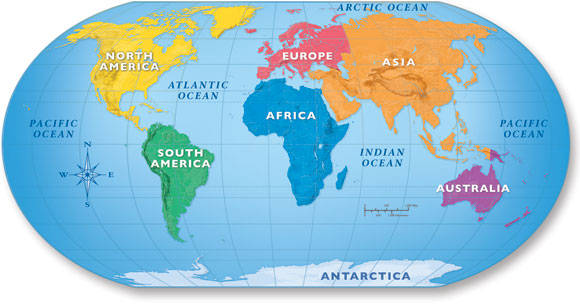
Geographical Footprint
MarPe covers all MENA and Africa having an especially strong presence in: Egypt, Jordon, Sudan, South Africa, Kenya, Burkina Faso, Oman, UAE, Lebanon & Saudi Arabia
More Details
MarPe's Network
-Clinical Labs: 1 - 5 clinical laboratories per country. -Depots: 1 - 3 pharmaceutical depots per country to store medical and clinical trial supplies. -Research Units: Clinical Research Units in university & governmental hospitals, with established infrastructure and qualified teams. -KOLs: Excellent relationships with the most experienced KOLs and their teams.
More Details

Advisory Boards
Our professional team of Medical writers can prepare and host these meetings if virtual, write meeting reports, presentations, and publications.
More Details
Data Management
Data Management Plan
Develop, Program & Test Automated Edit Checks
Database Setup
Biostatistics
MarPe’s statisticians analyse and present clinical data using statistical methodologies compliant with ICH E9 (R1) & E3. Our services range from protocol design to dissemination of results across all therapeutic areas.
More Details
Bioequivalence studies
Through our network of Bioequivalence centers, we manage bioequivalence studies to ensure compliance with international and local regulations.
More Details

Training
Our trainers deliver trainings to investigational sites and clinical operations personnel, face-to-face or via webinars. Training topics include:
More Details
Consulting
Research Center establishment and operations
Quality Management System
Risk Management System
Bio-sample Procurement
Through our network of Clinical Research Units, Laboratories, and Hospitals, we provide bio-samples for research purposes obtaining the mandatory approvals by the local Regulatory Authority and consent of the sample donors.
More Details